Sunday, January 23, 2022
Monday, January 10, 2022
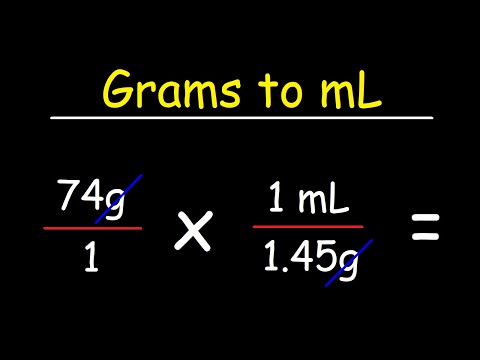
Density Of Air At 20 C In G Ml
During most operations, the direct measurement of the volume of blood collected in suction canisters is the most common clinical method used to determine intraoperative blood loss. When the volume of blood loss is small, the gravimetric method is a simple, accurate, and clinically relevant measurement technique . With this method, surgical gauze sponges and laparotomy pads are weighed before and after use. The difference in their weight is generally believed to be an accurate measurement of blood loss. To convert the mass of blood to a more familiar volume statistic, knowledge of blood density is required. The density of blood is generally estimated to be one gram per milliliter [2, 7–9].
However, there is a paucity of documentation that the one milliliter of blood weighs one gram relationship is accurate or if it holds true for varying hematocrits. Existing data are conflicting and demonstrate blood density ranges between 1043 and 1060 kg/m3 (1.043–1.060 g/mL) . The current study aims to determine the density of blood in an animal model and correlate it with the hematocrit. This will test the assumption that the specific gravity of blood is equal to water. If the relationship is valid and does not vary with hematocrit, it confirms the accuracy of weighing surgical sponges to determine intraoperative blood loss. For a better understanding of how temperature and pressure influence air density, let's focus on a case of dry air.
It contains mostly molecules of nitrogen and oxygen that are moving around at incredible speeds. Use our particles velocity calculator to see how fast they can move! For example, the average speed of a nitrogen molecule with a mass of 14 u (u - unified atomic mass unit) at room temperature is about 670 m/s - two times faster than the speed of sound! Moreover, at higher temperatures, gas molecules further accelerate. As a result, they push harder against their surroundings, expanding the volume of the gas .
And the higher the volume with the same amount of particles, the lower the density. Therefore, air's density decreases as the air is heated. A common use of Equation 10.23 is to determine the molar mass of an unknown gas by measuring its density at a known temperature and pressure. This method is particularly useful in identifying a gas that has been produced in a reaction, and it is not difficult to carry out. A flask or glass bulb of known volume is carefully dried, evacuated, sealed, and weighed empty.
It is then filled with a sample of a gas at a known temperature and pressure and reweighed. The difference in mass between the two readings is the mass of the gas. The volume of the flask is usually determined by weighing the flask when empty and when filled with a liquid of known density such as water.
The use of density measurements to calculate molar masses is illustrated in Example 10. Air density equations Air contains a mixture of dry air and water vapor. The amount of water vapor is a function of the relative humidity; it is also related to the dew point temperature of the air. If you need to calculate the density of dry air, you can apply the ideal gas law. This law expresses density as a function of temperature and pressure.
Like all gas laws, it is an approximation where real gases are concerned but is very good at low pressures and temperatures. Increasing temperature and pressure adds error to the calculation. An online air density calculator such as the one by Engineering Toolbox let you calculate theoretical values for air density at given temperatures and pressures. The website also provides an air density table of values at different temperatures and pressures. These graphs show how density and specific weight decrease at higher values of temperature and pressure. The density of air is usually denoted by the Greek letter ρ, and it measures the mass of air per unit volume (e.g. g / m3).
Dry air mostly consists of nitrogen (~78 %) and oxygen (~21 %). The remaining 1 % contains many different gases, among others, argon, carbon dioxide, neon or helium. However, the air will cease to be dry air when water vapor appears. Although the two terms often are used interchangeably, there is a technical difference between specific gravity and density.
Density is defined as the mass per unit volume of a substance. When the specific gravity is defined based on water at 4°C, then the specific gravity is equal to the density of the liquid. However, if the specific gravity is expressed at different temperatures, it will no longer be equal to the density. Although there is a difference between specific gravity and density, for the most part the values are similar enough to be used interchangeably in most situations. What two things do you need to know in order to find the density of water?
Students should realize that they need both the volume and mass of a sample of water to find its density. Suggest that students use a graduated cylinder to measure volume in milliliters. Suggest that students use a balance to measure the mass in grams.
Tell students that they can find mass by weighing the water. However, since water is a liquid, it needs to be in some sort of container. So in order to weigh the water, they have to weigh the container, too. Explain to students that they will have to subtract the mass of an empty graduated cylinder from the mass of the cylinder and water to get the mass of just the water. The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables . Some applications are illustrated in the following examples.
The approach used throughout is always to start with the same equation—the ideal gas law—and then determine which quantities are given and which need to be calculated. Let's begin with simple cases in which we are given three of the four parameters needed for a complete physical description of a gaseous sample. The density of air depends on many factors and can vary in different places. It mainly changes with temperature, relative humidity, pressure and hence with altitude . The air pressure can be related to the weight of the air over a given location.
It is easy to imagine that the higher you stand, the less air is above you and the pressure is lower (check out our definition of pressure!). Therefore, air pressure decreases with increasing altitude. In the following text, you will find out what is the air density at sea level and the standard air density.
The purpose of this study was to validate the commonly held assumption that the density of blood is one gram per milliliter. We found the average density of blood was 0.994 g/mL ± 0.032 g. Notably, changes in the hematocrit did not affect the density of the blood samples and analysis revealed no relationship between the two parameters.
Online calculator, figures and tables showing density and specific weight of oxygen, O2, at varying temperature and pressure - Imperial and SI Units. The density of air is the mass per unit volume of atmospheric gases. The density of air, or how light it is, depends on the temperature and pressure of the air.
Typically, the value given for the density of air is at STP . For liquids, mass density is not a strong function of temperature and pressure. Hence, density is generally assumed to be independent of the temperature and pressure for liquids. On the other hand, for gases, density varies with both temperature and pressure. The relation between ρ, T and P for gases is given by the ideal gas law, which will be presented in a later section.
The specific gravity is a ratio between the mass of a given volume and the mass of an equal volume of water at 4oC. Since the density of water at 4oC is practically 1.00 g/cc, the density and specific gravity of aqueous solutions are almost identical. The difference needs always to be considered for analytical work of high precision, however. Argon - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of argon, Ar, at varying temperature and pressure - Imperial and SI Units.
To get a better idea of the density of air specifically, you need to account for how air is made of different gases when formulating its density. At a constant temperature, pressure and volume, dry air is typically made of 78% nitrogen (N2), 21% oxygen (O2) and one percent argon (Ar). Note that every molecule listed is heavier that or equal to 18 u. Now, let's add some water vapor molecules to the gas with the total atomic weight of 18 u (H₂O - two atoms of hydrogen 1 u and one oxygen 16 u). According to Avogadro's law, the total number of molecules remains the same in the container under the same conditions . It means that water vapor molecules have to replace nitrogen, oxygen or argon.
Because molecules of H₂O are lighter than the other gases, the total mass of the gas decreases, decreasing the density of the air too. The measured mass of blood is nearly equal to distilled water. This confirms the assumption that the densities of blood and of distilled water are nearly equivalent. As the salt concentration increases, both maximum density and the freezing point will decrease 14. Average seawater has a salinity level of 35 PPT and has a shifted maximum density of -3.5°C 14.
This is more than a 7° difference from freshwater and is below seawater's freezing point of 1.9°C 14. Instead, the process on convection simply circulates the cooling water until the entire surface water column reaches the freezing point 42. As the phase boundary between liquid and solid requires the proper pressure as well as temperature, ice only beings to form on the surface 30. The density of moist air is calculated as the sum of the density of the dry air component of the mixture plus the density of the saturated component of the mixture.
In the first calculator, the vapour pressure of water vapour in saturated air at the nominated temperature is calculated and multiplied by the relative humidity to give the actual water vapour pressure. The water vapour pressure is then subtracted from the total pressure to give the pressure of the dry component of the parcel. Densities of the two components are then calculated and summed to give the final answer. Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3.
However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects. Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3). The calculator below can be used to estimate the density and specific weight of gaseous oxygen at given temperature and pressure. Though it may seem like nothing, the air around you has a density.
The density of air can be measured and studied for features of physics and chemistry such as its weight, mass or volume. The fact that they are listed on a per amount basis is an indication that the quantity of heat required to raise the temperature of a substance depends on how much substance there is. Any person who has boiled a pot of water on a stove, undoubtedly know this truth. Water boils at 100°C at sea level and at slightly lowered temperatures at higher elevations. To bring a pot of water to a boil, its temperature must first be raised to 100°C.
This temperature change is achieved by the absorption of heat from the stove burner. One quickly notices that it takes considerably more time to bring a full pot of water to a boil than to bring a half-full of water to a boil. This is because the full pot of water must absorb more heat to result in the same temperature change.
In fact, it requires twice as much heat to cause the same temperature change in twice the mass of water. Refers to the amount of heat required to cause a unit of mass to change its temperature by 1°C. Specific heat capacities of various materials are often listed in textbooks. Standard metric units are Joules/kilogram/Kelvin (J/kg/K). Use the widget below to view specific heat capacities of various materials. Simply type in the name of a substance (aluminum, iron, copper, water, methanol, wood, etc.) and click on the Submit button; results will be displayed in a separate window.
Water temperature and water density are directly related. As the temperature of water increases or decreases, it will alter the density of water. This is a unique relationship in that unlike most materials, the density of pure water decreases approximately 9% when it freezes 29. Pure water is also unique in that it achieves its maximum density, 1.00 g/ml, at 4°C 29.
Water at temperatures above and below this, including superheated and supercooled water, will float on 4°C water. Water temperature is a physical property expressing how hot or cold water is. As hot and cold are both arbitrary terms, temperature can further be defined as a measurement of the average thermal energy of a substance 5. Thermal energy is the kinetic energy of atoms and molecules, so temperature in turn measures the average kinetic energy of the atoms and molecules 5. This energy can be transferred between substances as the flow of heat. Heat transfer, whether from the air, sunlight, another water source or thermal pollution can change the temperature of water.
The specific gravity of a liquid is the relative weight of that liquid compared to an equal volume of water. Liquids that are lighter than water have a specific gravity less than 1 and those heavier than water have a specific gravity greater than 1. Specific gravity is dependent on the temperature, and most of the values found in the literature refer to STP conditions.
The soil bulk density , also known as dry bulk density, is the weight of dry soil divided by the total soil volume . The total soil volume is the combined volume of solids and pores which may contain air or water , or both . The average values of air, water and solid in soil are easily measured and are a useful indication of a soils physical condition. Is defined as a hypothetical gaseous substance whose behavior is independent of attractive and repulsive forces and can be completely described by the ideal gas law. In reality, there is no such thing as an ideal gas, but an ideal gas is a useful conceptual model that allows us to understand how gases respond to changing conditions.
As we shall see, under many conditions, most real gases exhibit behavior that closely approximates that of an ideal gas. The ideal gas law can therefore be used to predict the behavior of real gases under most conditions. The buoyancy correction for a solid is small, and frequently ignored. It may be significant, however, for low density liquids and gases. This is particularly important when calibrating glassware.
Medium Seaaborn Mathplot Diesign Styles
Allows you to draw a grid of small subplots where each row and column corresponds to a different variable. The resulting grid displays all t...

-
Allows you to draw a grid of small subplots where each row and column corresponds to a different variable. The resulting grid displays all t...
-
During most operations, the direct measurement of the volume of blood collected in suction canisters is the most common clinical method used...
-
Empty Message